3 Reasons You Should not Teach Ionic Bonding as Electron Transfer
- Androy
- Apr 9, 2022
- 6 min read
Updated: May 20, 2024
The convention of teaching ionic bonding as an electron transfer event has no relevance to any likely chemical process and has little to do with ionic bonding. In this post, I discuss three ways teaching ionic bonding as electron transfer can be problematic.

In How the Misuse of the Octet Rule leads to Misconceptions in Chemistry we discussed how students’ focus on the full shell or octet structure can lead to a wide variety of misconceptions related to chemical bonding.
Yet, it is clear that many textbook diagrams and explanations perpetuate these ideas and encourage learners to think in terms of chemical reactions which: occur between isolated atoms that are actively seeking electrons to fill their shells in order to achieve stability.
Ionic bonding is similarly understood as a metal transferring a valence electron to a nonmetal in order to have a full outer shell and to achieve stability.
This convention of teaching ionic bonding as an electron transfer event has no relevance to any likely chemical process and has little to do with ionic bonds and the properties of ionic compounds.
So, with that said, here are THREE reasons you should not teach ionic bonding as electron transfer.
Reason 1:
Electron transfer has nothing to do with ionic bonding
The diagrams typically presented in students' textbooks often depict an atom (sodium) transferring its valence electron to a chlorine atom, resulting in the formation and then the interaction of the resulting ions to form an ion-pair, NaCl.

Why is this depiction problematic?
1. Students often believe this is how ionic bonds are formed and some even come to believe that this transfer of electron(s) is in itself the ionic bond.
This scheme does not represent a likely chemical event and was most likely developed as a pedagogical tool to explain a complex chemical concept.
Ions are readily available in our environment in the form of salts no electron transfer event was necessary.
Because of the emphasis placed on the transfer of electrons in our classrooms and textbook diagrams, students will often define ionic bonding as " the transfer of electrons" and little value is given to the electrical interactions between oppositely charged ions which is essentially what the ionic bond is.
According to Taber (2012), the transfer of electron depiction is a "thought experiment" on what might occur if these reactions started from individual atoms and is completely irrelevant to ionic bonding and the properties of ionic substances.
2. Students are led to believe that these reactions such as the formation of sodium chloride begin with isolated atoms of sodium and chlorine.
Chemical reactions rarely occur between isolated atoms. In fact, very little of the matter which exists on earth is in the form of isolated atoms (the noble gasses being the exception); therefore chemical bonding should not be taught in this way.
The formation of sodium chloride does NOT begin with a sodium atom and a chlorine atom.
There is no electron transfer during ionic bond formation.
Taber (2012) suggests that it is more useful to introduce students to ionic bonding using ionic precipitation reactions or neutralization reactions as they are able to see the formation of the ionic product immediately. This organically leads to discussions on ionic lattices.
The idea of electron transfer may have started as a pedagogical tool, but Joki et al. (2015) argue that the models taught should be as accurate as possible from the onset so there is little to unlearn during later grades.

Reason 2:
Students believe that ionic bonding occurs so that atoms can become stable
Students use the octet/full shell framework to explain why chemical bonds form and as a reason for the chemical stability of the many compounds they encounter during their school careers.
By extension, students similarly conceptualize ionic bonding this way. The metal transfers its electron to the nonmetal so that it has a full outer shell.
To test your students' misconceptions related to the octet rule try this diagnostic probe.
Why is this type of thinking problematic?
1. Chemical stability is a thermodynamic concept the octet rule and electronic configuration should not be used as an explanation for chemical stability.
You can read more about this here.
2. Students will attempt to fit all chemical reactions into this electron transfer framework.
Research shows that students erroneously believe that atoms maintain their discreet identity within molecules or lattice structures so that bonding electrons are still considered to belong to the atom from which they originated (Joki et al. 2015 ;Taber, 2002, 2012).
This idea appeals to students so much that they often have difficulty understanding heterolytic fissions and some students also believe that for a precipitation reaction to occur that the electron(s) must first return to their original atoms before new ionic compounds can be formed.
Taber (2012) showed that when students were asked to explain the precipitation for silver chloride by the reaction of aqueous sodium chloride and aqueous silver nitrate, students did so by stating that:
the nitrate ion must first return the electron to the silver ion
the sodium must get its electron back from the chloride ion,
the silver atom will then donate this electron to the chlorine atom to form silver chloride.
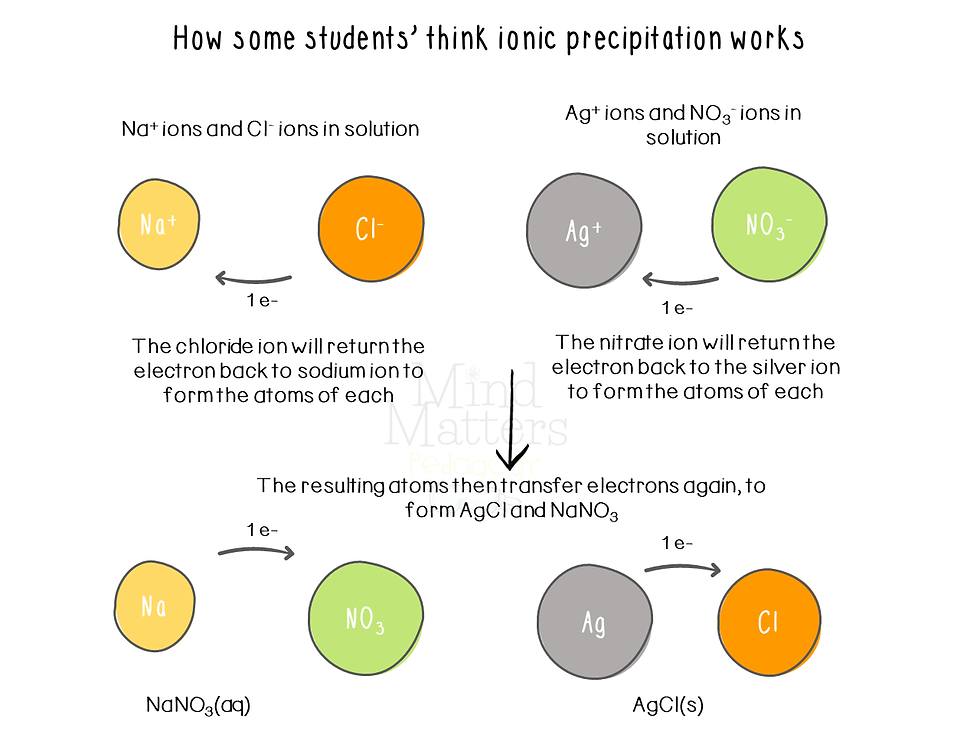
However, we know that these ions already exist in the aqueous solutions. The strong attraction between the silver ions and the chloride ions, causes them to clump together to eventually form the ionic lattice of silver chloride (the precipitate).
Reason 3:
Students see ionic substances as being made up of ion pairs or molecules
As mentioned earlier, textbook diagrams have a tendency to focus on depicting the transfer of electrons from a metal to a non-metal resulting in an ion pair. As a result of this representation ionic compounds are seen as having molecule-like status which Taber (2002) refers to as the molecular framework. Even when students know that ionic substances have lattice structures, they still imagine these structures to be made up of pairs of ions.
Why is this problematic?
1. Research shows that students who are taught ionic bonding with the focus on electron transfer rather than electrical interactions tend to believe that the valency or ionic charge determines the number of ionic bonds formed
For example, when presented with ionic bonding in Lithium fluoride a student would determine that a lithium atom can only donate one electron and will therefore form only one ionic bond with another fluoride ion, when in fact the number of bonds formed depends on the arrangement of the ions in the lattice structure i.e. the coordination number.
Similarly, when presented with a cross-section of the sodium chloride lattice some students determined that each sodium ion belonged to or was bonded to the chloride ion to which it donated its electron.
2. Students often take the lattice structure of ionic substances for granted
Because of students' idea of the "ion-pair", they often become confused when discussing the properties of ionic substances such as melting point/boiling point, solubility, hardness, etc. since the molecular framework does not lend itself to a proper explanation of these concepts.
Like all chemical bonds, the ionic bond is as a result of electrical interactions, this concept alone is enough to fully explain :
the structure of ionic compounds
the physical properties of ionic substances such as melting point etc.

Tips for teachers:
Teaching how ions are formed is unnecessary, instead, explore the electrostatic interactions of the subatomic particles and have students figure out the differences between the atoms and ions of an element.
Focus on the electrical interactions when teaching chemical bonding and move away from the irrelevant electron transfer concept, which only reinforces misconceptions.
When introducing ionic bonding to students it is more useful to think in terms of more feasible chemical reactions such as precipitation reactions which students will more than likely be exposed to during their school careers.


References
Joki, Jarkko, et al. (2015) “Coulombic interaction in Finnish Middle school Chemistry: a systematic perspective on students' conceptual structure of chemical bonding.” Chemistry Education Research and Practice.
Joki, J., & Aksela, M. (2018). The challenges of learning and teaching chemical bonding at different school levels using electrostatic interactions instead of the octet rule as a teaching model. Chemistry Education Research and Practice, 19(3), 923. https://pubs.rsc.org/en/content/articlelanding/2018/RP/C8RP00110C#cit7
Taber, K. (Ed.). (2012). Teaching Secondary Chemistry. Hodder Education.
Taber, K. S. (1998). An Alternative Conceptual framework from Chemistry education. International Journal of Science Education, 20(5), 597-608.
Taber, K. S. (2002). Chemical Misconceptions--Prevention diagnosis and cure. Vol.1: Theoretical Back Ground. London: Royal Society of Chemistry.
Taber, K. S. (2005). Learning Quanta: barriers to stimulating transitions in student understanding of orbital ideas. Science Education, 42, 125-184.
Taber, K. S. (2009). College students' conceptions of chemical stability: the widespread adoption of a heuristic rule out of context and beyond its range of application. International Journal of Science Education, 31, 1333-1358.








Comments